[GUEST ACCESS MODE: Data is scrambled or limited to provide examples. Make requests using your API key to unlock full data. Check https://lunarcrush.ai/auth for authentication information.]  Andrew Caravello, DO [@andrewcaravello](/creator/twitter/andrewcaravello) on x 1070 followers Created: 2025-07-17 17:31:54 UTC Great question, and you are right to sense that this sits somewhere between $NWBO DCVax-L and Direct in format. In the Mayo trial, they used autologous dendritic cells, meaning each patient’s own immune cells were collected via leukapheresis. But instead of harvesting tumor tissue from each patient like in DCVax-L, they pulsed those dendritic cells with a pooled tumor lysate library, made from surgically resected glioblastoma tumors donated by other patients. That lysate was processed under GMP conditions into a standardized cryopreserved product. It was almost certainly manufactured and stored by Mill Creek Life Sciences, a Mayo Clinic spinout that specializes in lysate preparation and clinical grade media for cell therapies. Their bank of pooled GBM lysate served as the antigen source across all participants. After pulsing the dendritic cells with this shared antigen library, they reinjected them intradermally, not into the tumor. So there was no need for tumor surgery and no intratumoral injection like DCVax Direct. The treatment was personalized through the dendritic cells, not the antigen source. The result is a platform that maintains all the critical features of DCVax, including autologous dendritic cells, ex vivo maturation using the patented Northwest Biotherapeutics process, and precise immune activation. The antigens were allogeneic and standardized, but the immune response remained patient specific because the dendritic cells were not shared. So yes, this is a hybrid using simplified delivery, pooled lysate, and no surgery, but it is still built entirely on the DCVax engine. The dendritic cell is the instructor. The lysate is just the curriculum. 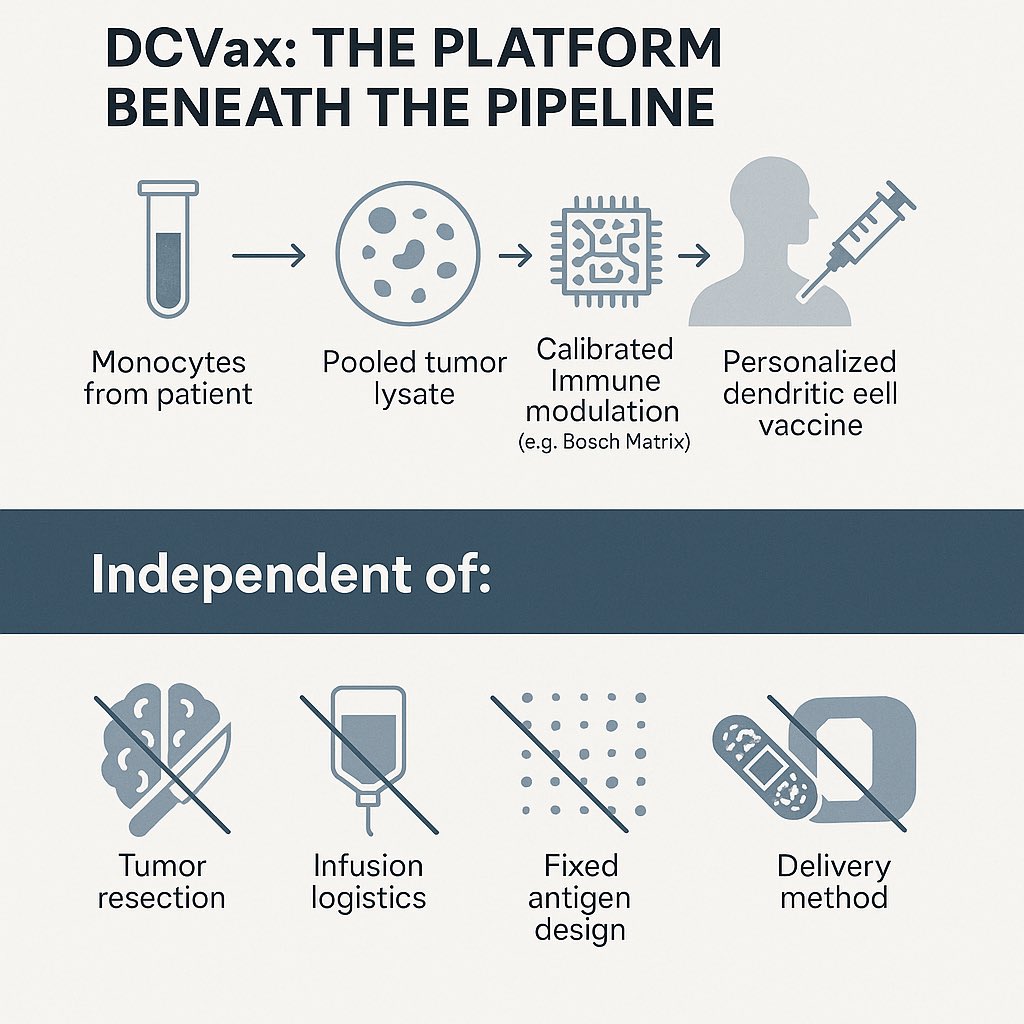 XXX engagements  **Related Topics** [$nwbo](/topic/$nwbo) [Post Link](https://x.com/andrewcaravello/status/1945899271615815705)
[GUEST ACCESS MODE: Data is scrambled or limited to provide examples. Make requests using your API key to unlock full data. Check https://lunarcrush.ai/auth for authentication information.]
 Andrew Caravello, DO @andrewcaravello on x 1070 followers
Created: 2025-07-17 17:31:54 UTC
Andrew Caravello, DO @andrewcaravello on x 1070 followers
Created: 2025-07-17 17:31:54 UTC
Great question, and you are right to sense that this sits somewhere between $NWBO DCVax-L and Direct in format.
In the Mayo trial, they used autologous dendritic cells, meaning each patient’s own immune cells were collected via leukapheresis. But instead of harvesting tumor tissue from each patient like in DCVax-L, they pulsed those dendritic cells with a pooled tumor lysate library, made from surgically resected glioblastoma tumors donated by other patients.
That lysate was processed under GMP conditions into a standardized cryopreserved product. It was almost certainly manufactured and stored by Mill Creek Life Sciences, a Mayo Clinic spinout that specializes in lysate preparation and clinical grade media for cell therapies. Their bank of pooled GBM lysate served as the antigen source across all participants.
After pulsing the dendritic cells with this shared antigen library, they reinjected them intradermally, not into the tumor. So there was no need for tumor surgery and no intratumoral injection like DCVax Direct. The treatment was personalized through the dendritic cells, not the antigen source.
The result is a platform that maintains all the critical features of DCVax, including autologous dendritic cells, ex vivo maturation using the patented Northwest Biotherapeutics process, and precise immune activation. The antigens were allogeneic and standardized, but the immune response remained patient specific because the dendritic cells were not shared.
So yes, this is a hybrid using simplified delivery, pooled lysate, and no surgery, but it is still built entirely on the DCVax engine. The dendritic cell is the instructor. The lysate is just the curriculum.

XXX engagements
Related Topics $nwbo