[GUEST ACCESS MODE: Data is scrambled or limited to provide examples. Make requests using your API key to unlock full data. Check https://lunarcrush.ai/auth for authentication information.]  Andrew Caravello, DO [@andrewcaravello](/creator/twitter/andrewcaravello) on x 1071 followers Created: 2025-07-14 08:56:09 UTC 🧠Strategic Convergence: Yorkville, $NWBO, Indaptus, and Modular Immune Architecture 📡 TL;DR (22-25 min read): Yorkville’s dual financing of NWBO and $INDP reveals a deeper strategy: the rise of a programmable immunotherapy system. #DCVax, DECOY20, and Eden form the core of an adaptive platform that integrates danger signals, viral ignition, and synthetic TLR boosters. Not a drug; a system. Not a company; a platform. In early 2025, Yorkville Advisors (YA II PN, Ltd.) executed two sequential financing agreements: X. A $X million convertible note with Northwest Biotherapeutics, announced on February 13, 2025 X. A standby equity purchase agreement with Indaptus Therapeutics (INDP), publicly disclosed shortly after These deals weren’t random. Yorkville’s dual investment gives it direct exposure to two sides of the same immunologic loop: • NWBO, which owns the immune delivery system (DCVax + Eden) • Indaptus, which supplies the microbial immune booster (DECOY10/20) In May 2025, Indaptus further announced a new offering and public company presentation highlighting the mechanism of action for DECOY20 and its clinical trial pipeline, including NCT05651022. With Yorkville holding a significant equity position and purchasing rights through the new financing structure, it effectively owns a pathway into the “danger signal” component of NWBO’s therapeutic matrix. The strategic implication is clear: Yorkville is not a passive financier. It now holds a personal financial stake in one of NWBO’s most viable immune boosting agents. From a capital structure perspective, this alignment supports: • Internal licensing flexibility between companies • Optional coordinated deployment under Specials • Future positioning for regulatory linked immune signal expansion 🧬 NWBO: Platform and Delivery NWBO has developed DCVax, a dendritic cell based immunotherapy platform protected by exclusive patents and licenses covering the preparation, maturation, and administration of autologous dendritic cells. It trains the immune system using tumor lysate, most commonly autologous in DCVax-L. Exploratory trials using pooled or shared lysate libraries have been studied separately, but are not part of the DCVax-L platform. Its safety and systemic activation profile have been validated in Phase X glioblastoma trials led by Dr. Linda Liau. In 2020, NWBO acquired Flaskworks and with it, the Eden platform, a closed system, sensor equipped cell therapy manufacturing unit designed for decentralized, GMP compliant, point of care deployment. Eden allows for: • Automated, programmable DCVax production • Modular input selection (lysate, boosters, synthetic payloads) • Regulatory adaptability under frameworks like the UK’s SI XX NWBO’s system is not a static treatment. It is a responsive, immune interface. From the NYAS 2025 presentation, Dr. Marnix Bosch introduced a booster code matrix (e.g., IAV, IABD, IAVCBD) that maps to cytokine outputs, immune targets, and site specific strategies, designed to enable modular therapy based on tumor type, booster compatibility, and clinical setting. This labeling logic, if paired with Eden, transforms DCVax from a fixed product into an adaptable immunologic framework. The Bosch presentation served as both a biological mechanism map and a regulatory calibration system. Because each code corresponds to a predefined immune profile, NWBO can now deliver different variants of DCVax under real world conditions without submitting an entirely new marketing authorization each time. The labeling becomes functional and adaptive. Calibration replaces rigidity. This essentially makes DCVax universal, applicable across tissue types, indications, and delivery sites. 💥 Indaptus: Danger Signal Amplification Indaptus Therapeutics is developing DECOY10 and DECOY20, killed, nonreplicating strains of E. coli engineered to mimic microbial danger signals. Their mechanism of action includes TLR4 and NFκB activation, type I interferon production, and broad cytokine release across innate and adaptive compartments. These constructs are designed not as therapies but as immune ignition payloads that provoke systemic immune activation. DECOY agents simulate infection through bacterial pattern recognition receptor pathways, triggering innate and adaptive responses. In studies presented at AACR 2024, SITC 2023, and published in Frontiers in Immunology (2024), DECOY10 consistently outperformed monospecific TLR agonists like Poly I C, R848, CpG, and LPS across key cytokines: GM CSF, IFNγ, IL 12p70, TNFα, and MIP1α. This cytokine signature has now been clinically validated. In 2025, Indaptus completed the validation phase of its Phase 1b/2 trial (NCT05651022), evaluating DECOY20 in combination with BeiGene’s PD X inhibitor Tislelizumab. The trial confirmed immune activation and cytokine induction at therapeutically relevant levels with a clean safety profile. DECOY20 is now advancing to the next stage as a clinically qualified, system ready D class input. This assignment directly maps to the Bosch booster matrix, introduced during NWBO’s NYAS 2025 presentation, which categorizes immune adjuvants as follows: A class: Viral mimics (e.g. Poly I C, TLR3) B class: TLR4 ligands (e.g. G100, synthetic lipid A) C class: TLR7/8 agonists (e.g. R848) D class: Microbial danger signals (e.g. DECOY10/20) I class: Immune tone recalibrators (e.g. IFNγ) V class: Viral ignition (e.g. V937) DECOY10’s cytokine output spans Bosch’s entire logic grid: GM CSF for dendritic cell maturation, IFNγ and IL 12p70 for T cell polarization and cytotoxic programming, TNFα for inflammation and tumor antigen release, and MIP1α for APC and lymphocyte recruitment. Functionally, DECOY10 extends well beyond the D class, overlapping A, B, C, and I booster functions. This cross-functional profile is what makes DECOY uniquely suited for Eden, NWBO’s closed-system, programmable cell therapy manufacturing unit. Eden allows real time customization of DCVax formulations by site and patient using modular combinations like IAV, IABD, or IAVCBD. DECOY10 could simplify this structure by collapsing multiple inputs into a single cartridge, streamlining operations while preserving regulatory continuity under frameworks like SI XX. Yorkville Advisors appears to have anticipated this. In July 2025, following Indaptus’ trial milestone, Yorkville provided an additional XXX million dollars via convertible note, with a X percent interest rate, XXX percent warrant coverage, and a XX month maturity. Full terms were disclosed here: This was not bridge capital. It was strategic positioning. Yorkville had already financed NWBO in February 2025. Now, with equity exposure to both Indaptus and NWBO, Yorkville controls both ignition and delivery. According to market participants, Yorkville may convert early to secure equity ahead of broader institutional recognition. That urgency reflects conviction. DECOY20 is no longer a development-stage adjuvant. It is now an activated, clinically validated booster aligned with Eden’s real world deployment logic. Yorkville’s NWBO note further supports this. It was structured not as secured debt, but as a convertible position, implying confidence in NWBO’s regulatory and commercial trajectory. The willingness to hold equity rather than debt, combined with the Indaptus structure, positions Yorkville across the entire immunotherapy system: signal (DECOY), engine (Eden), and output (DCVax). With Eden’s programmable chassis, DECOY10 and DECOY20 can be deployed not only as D class inputs, but functionally across IAV, DIV, or IABD-coded formulations. In cold tumors, fibrotic lesions, or inoperable sites, DECOY’s multipathway output can replace multiagent regimens with a single, site-customized immune ignition. This is not a single company story. It is a coordinated platform architecture. DECOY delivers the signal. Eden executes the calibration. DCVax delivers the outcome. Yorkville is positioned across all three. 🧪 Merck’s V937: Viral Signal and Oncolytic Synergy Merck’s V937 (Coxsackievirus A21) is a live, replication competent oncolytic virus designed to lyse tumor cells and stimulate local and systemic immune activation. It was acquired by Merck through its $XXX million purchase of Viralytics. V937’s mechanism of action includes: • Direct tumor cell lysis • Release of tumor associated antigens • Activation of pathogen recognition receptors including RIG I, MDA5, and TLR3 • Induction of type I interferon signaling • Promotion of immunogenic cell death (ICD) through release of DAMPs such as dsRNA, HMGB1, ATP, and calreticulin V937 has been tested in multiple combination trials with pembrolizumab (Keytruda) in solid tumors (e.g. NCT02565992, NCT03323492), where it has shown the ability to transform cold tumors into immunologically active environments. Importantly, NWBO’s XX K disclosed a Material Transfer Agreement in place since mid 2024 for a booster agent developed by a large pharmaceutical company, currently undergoing internal evaluation. The timing, classification, and delivery logic make V937 a strong candidate for this role. V937 remains under Merck’s control, and any use by NWBO would likely follow a licensing or limited scope agreement under Specials or future combination trials. As a V class booster in NWBO’s framework, V937 could serve to precondition tumors prior to DCVax Direct injection, amplifying antigen spread, recruiting antigen presenting cells, and enhancing dendritic cell priming in vivo. 🧩 Functional Roles in NWBO’s Booster Matrix From NWBO’s NYAS 2025 presentation, Slide X outlines a modular classification of dendritic cell booster types that can be combined in programmable sequences: • A, B, C: TLR ligands • I: Immune modulators (e.g., IFNγ, checkpoint pathway agents) • D: Danger signals (e.g., DECOY10/20) • V: Viral pattern mimics (e.g., Merck’s V937) These categories are deployed singly or in rational combinations (e.g., IAV, IABD, IAVCBD) to enhance specific immune functions. Each code combination corresponds to a unique immunological strategy: increasing TNFα and IL-12p70, shifting T cell polarization, promoting chemotaxis, or overriding tumor-induced suppression. 🎯 A B C Now Explicitly Mapped (Easy as X X 3) The A, B, and C classes are now identifiable and anchored in both immunology and industry lineage: • A = Poly I:C (TLR3): A synthetic double-stranded RNA analog that mimics viral infection and drives type I interferon signaling through TLR3. Its most clinically relevant form is Poly ICLC, stabilized with carboxymethylcellulose and poly-L-lysine. This formulation is known commercially as Hiltonol, developed and manufactured by Oncovir, Inc. Hiltonol has been used in numerous dendritic cell vaccine trials, including the Phase X glioblastoma combination trial with DCVax-L, whose results were published in Nature Communications in March 2024. That study showed a greater than XX% survival benefit for newly diagnosed glioblastoma patients treated with DCVax-L plus Hiltonol compared to historical controls. It validated the systemic priming power of Poly ICLC and reinforced the cytokine logic behind Bosch’s IAV/IABD-type booster profiles. • B = G100 (TLR4): A synthetic lipid A analog that activates TLR4 with significantly reduced toxicity compared to LPS. G100 was developed by Immune Design and acquired by Merck & Co. It has been used in multiple early-phase cancer trials, particularly intratumorally in lymphoma, and is known for driving local cytokine production and dendritic cell activation in vivo. Its inclusion in NWBO’s Eden-compatible matrix suggests Merck-derived TLR4 logic is interoperable with DCVax-Direct formulations, particularly in D/V/A-coded combinations. • C = R848 (Resiquimod, TLR7/8): R848 is a potent imidazoquinoline compound that activates TLR7 and TLR8, primarily in monocytes, dendritic cells, and macrophages. It was developed by MedImmune and is now owned by AstraZeneca. R848 has been used as a topical immune stimulant and as an injectable adjuvant in preclinical and early-phase cancer immunotherapy. It induces IFNα, IL-12, and TNFα and plays a complementary role to Poly I:C by targeting different subsets of innate cells. Its Th1-skewing and dendritic cell-activating effects make it a natural fit for Eden’s programmable IAV/IAC-type profiles, especially in combination with danger signals (D) or checkpoint modulators (I). This classification is not arbitrary. These agents are not just immune potentiators, they are patented strategic assets. Their integration into NWBO’s Eden-compatible system implies molecular and operational interoperability with technologies controlled by Merck, AstraZeneca, Amgen, and Oncovir. If true, that represents a quiet but profound structural alignment: one that bridges next-generation immune activation with legacy pharma control points, all layered into a modular, programmable system that NWBO now appears to own. 🧪 Plug and Play Boosters: Merck (B class), AstraZeneca (C class), Amgen (I class) Merck: G100 (TLR4, B class) G100 is a synthetic lipid A analog that activates TLR4 with reduced toxicity. Originally developed by Immune Design and now owned by Merck, it has been used intratumorally in early phase oncology trials. In the Eden matrix, G100 serves as a B class booster and pairs well with microbial or immune modulatory co-signals. AstraZeneca: R848 (TLR7/8, C class) R848 (Resiquimod), acquired through AstraZeneca’s 2007 acquisition of MedImmune, is a potent TLR7/8 agonist capable of triggering IFN alpha, IL-12, and TNF alpha. It activates plasmacytoid dendritic cells and supports Th1 polarized immunity. As a C class input, R848 complements Poly I C by expanding Eden’s reach across innate sensing pathways. Amgen: Actimmune (IFNγ, I class) Actimmune is the GMP formulation of interferon gamma (IFNγ), originally developed by Horizon Therapeutics and now owned by Amgen. Approved for chronic granulomatous disease and osteopetrosis, it has been incorporated into immunotherapy combinations as a classical biological response modifier. In Bosch’s NYAS 2025 presentation, IFNγ was explicitly referenced in the “Rescue of Low Producers” slide, where it converted suboptimal dendritic cell responses into high output cytokine profiles. It defines the I class: immune tone modulation and dendritic cell reactivation. Each plug-and-play agent maps directly to Bosch’s A–I classification system, Merck’s G100 as B, AstraZeneca’s R848 as C, and Amgen’s IFNγ as I, demonstrating functional interoperability across the Eden framework. 🧬 Modular Architecture and Regulatory Significance Each class plays a role in immune engagement: • A B C: Synthetic innate immune activators, precisely titratable and GMP compatible • D: Microbial danger signals like DECOY10/20, broad spectrum activators of NFκB and type I IFN • V: Viral oncolytics like V937, releasing tumor antigens and danger signals in situ • I: Immune modulators that shape cytokine tone and checkpoint responsiveness (e.g., IFNγ, see Amgen’s Actimmune) What Bosch presented was more than a biology slide. It was a modular labeling system. The code matrix is designed to function within the UK’s SI XX framework, allowing real time formulation switching (per patient, site, or protocol) without resetting the regulatory baseline. In essence, DCVax has become relabelable by immune logic. This flexibility, paired with Eden’s ability to execute customized formulations at the point of care, turns NWBO’s therapy from a static product into a universal programmable immunologic framework. This modular framework depends on execution systems like Eden to ensure real-time precision and regulatory alignment, especially as global standards evolve. ⚙️ Flaskworks and Eden: The Integration Layer Flaskworks’ Eden system is not just a device. It is the execution layer of NWBO’s entire immunologic logic. While the booster matrix (A to I) defines the immune inputs, Eden delivers the output: patient-specific, GMP-grade DCVax formulations calibrated to site, tumor, and signal. It breaks the manufacturing bottleneck that has historically prevented cell therapy from scaling. More than that, it redefines the regulatory perimeter. Eden enables NWBO to swap in new boosters, adjust formulations, and apply localized immune strategy, all without reauthorizing the core DCVax platform. This is made possible by Eden’s unique features: • Per patient, per site, per booster configurability • Full traceability and immune signature documentation • Programmable logic built to match regulatory frameworks like SI XX • Rapid adaptation to local tumor immune environments (e.g., IAV at one site, IABD at another) Plugged into Eden, NWBO can now integrate: • DECOY20 as a D class microbial adjuvant (Indaptus) • V937 as a V class viral igniter (Merck) • G100 as B class (Merck) • R848 as C class (AstraZeneca) • Actimmune as I class (Amgen) Each of these once stood alone as an investigational adjuvant. Now, through Eden, they can become programmable subroutines in a living immunologic engine. That shift, from product to platform, is what makes Eden central not just to NWBO, but to the future of decentralized immunotherapy. 💼 Strategic Implications and Yorkville’s Position Yorkville is not just financing both companies. It has become one of the most prominent institutional holders of Indaptus and a direct capital partner to NWBO. With follow-on purchasing rights and exposure to the DECOY platform through Indaptus’ equity agreement, Yorkville has secured a cross-platform foothold: • It can influence or broker booster integration between DECOY and DCVax • It has visibility into both manufacturing logic and immune signal design • It has potential positioning leverage as Specials transition into trial-based programs This alignment gives Yorkville more than just capital exposure. It gives it strategic optionality across one of the most modular and programmable cell therapy architectures in oncology. What makes DECOY20 unique, compared to synthetic agonists or legacy immunomodulators, is its ability to deliver a complete microbial danger signal in a single payload. It combines TLR4 engagement, NFκB activation, and type I interferon release into one broad-spectrum immune ignition event. Where synthetic inputs like G100 or R848 act on specific receptors, DECOY20 triggers a cascade, mimicking natural pathogen cues and potentially awakening immune responses in tumors that resist conventional priming. This is likely why Yorkville chose to anchor itself here. DECOY20 is not an accessory; it’s a keystone signal. One that becomes exponentially more powerful when paired with a delivery engine like DCVax and a modular chassis like Eden. Would it make sense for Yorkville to become a large investor in Indaptus if DECOY20 had no downstream partner or clinical use case? Probably not. Its standalone activity is promising, but its systemic value emerges through integration. As Eden evolves to accommodate real-world input variation, DECOY20 becomes a dynamic programmable booster that plugs directly into a modular therapeutic engine. The value is not abstract. It is functional. And Yorkville is embedded on both ends of that function. And as for the old toxic loan trope, Yorkville’s behavior makes the opposite case. Its continued investment, the increasingly favorable terms of its most recent offering, and its cross-platform exposure suggest a partner that is betting on this ecosystem to scale, succeed, and lead. As Eden brings real-world adaptability to DCVax, DECOY20 becomes a live signal in a modular therapeutic engine. The value is not theoretical. It is functional. And Yorkville is positioned across both ends of that system. 🔚 Final Thought If DECOY20 is D, V937 is V, Eden is programmable, and the TLR class is now explicitly populated with Poly I:C (TLR3), G100 (TLR4), and R848 (TLR7/8), then NWBO is no longer competing over whose agent is superior. It is architecting the immune stack that lets them all connect. This is not a therapy built around a molecule. It is a programmable interface for immune logic, one that incorporates the best available danger signals, viral ignition tools, innate calibrators, and checkpoint enablers into patient-specific formulations. Each module engages the immune system from a distinct axis: • D class: microbial danger • V class: viral ignition • A B C class: synthetic innate agonists • I class: immune recalibration And all of it is controlled through Flaskworks’ Eden: a GMP-grade, traceable, and relabelable manufacturing platform designed not to produce one treatment, but to adapt many. It enables calibration by site, by signal, or by strategy, without resetting the regulatory baseline. The Bosch booster matrix is not just a scientific diagram. It is a control system: a regulatory schema that formalizes immune orchestration under frameworks like SI XX. In that context, NWBO isn’t competing with pharma. It is programming it. This is not a trial. It is a system. And Yorkville may already see it. Bosch’s presentation is the gift that keeps on giving.💎 #Flaskworks #EdenSystem #DCVax #DECOY20 #V937 #Immunotherapy #CellTherapy #BoschMatrix #ModularMedicine #PrecisionMedicine #RealWorldEvidence #CDMO #CheckpointTherapy #TME #PointOfCare $MRK $AMGN $AZN $ABBV $LLY $VRTX $AMZN 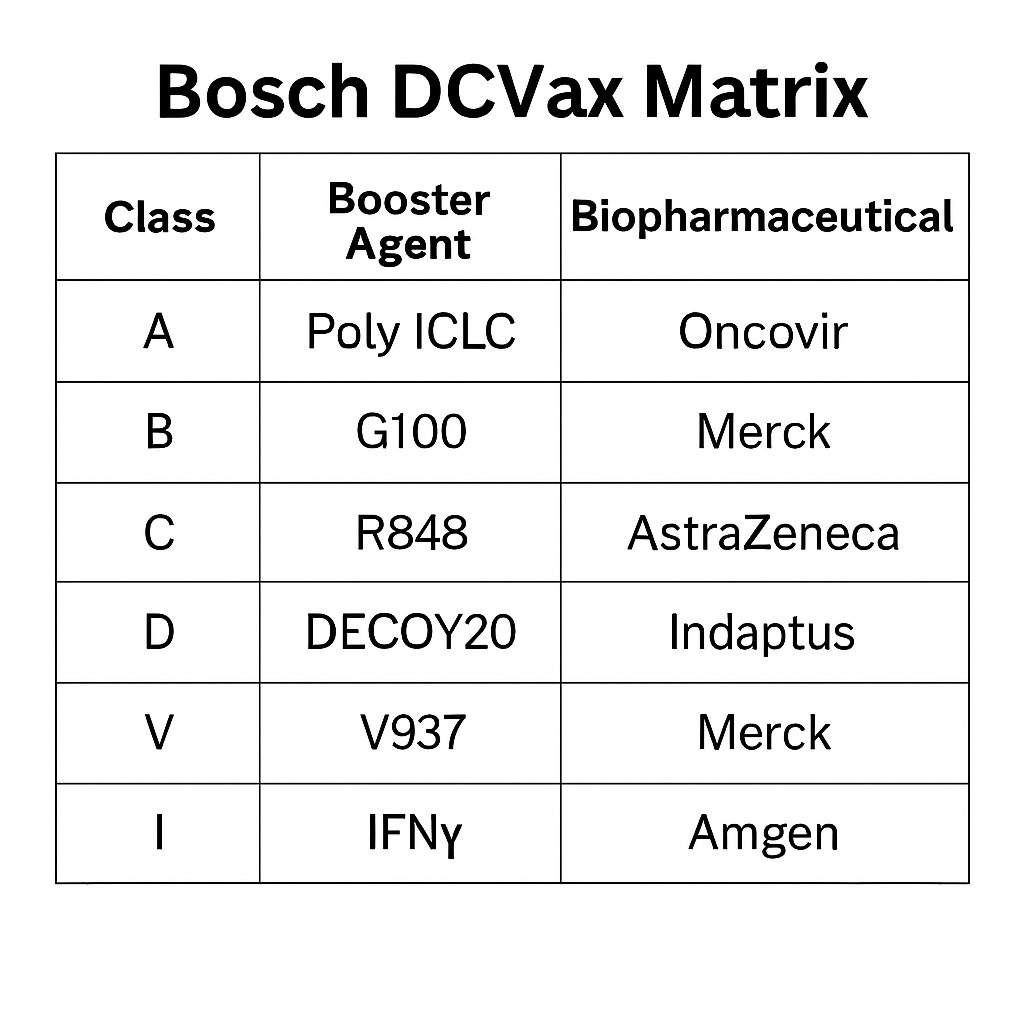 XXXXX engagements  **Related Topics** [signals](/topic/signals) [$indp](/topic/$indp) [$nwbo](/topic/$nwbo) [convergence](/topic/convergence) [Post Link](https://x.com/andrewcaravello/status/1944682317563277584)
[GUEST ACCESS MODE: Data is scrambled or limited to provide examples. Make requests using your API key to unlock full data. Check https://lunarcrush.ai/auth for authentication information.]
 Andrew Caravello, DO @andrewcaravello on x 1071 followers
Created: 2025-07-14 08:56:09 UTC
Andrew Caravello, DO @andrewcaravello on x 1071 followers
Created: 2025-07-14 08:56:09 UTC
🧠Strategic Convergence: Yorkville, $NWBO, Indaptus, and Modular Immune Architecture
📡 TL;DR (22-25 min read): Yorkville’s dual financing of NWBO and $INDP reveals a deeper strategy: the rise of a programmable immunotherapy system. #DCVax, DECOY20, and Eden form the core of an adaptive platform that integrates danger signals, viral ignition, and synthetic TLR boosters. Not a drug; a system. Not a company; a platform.
In early 2025, Yorkville Advisors (YA II PN, Ltd.) executed two sequential financing agreements:
X. A $X million convertible note with Northwest Biotherapeutics, announced on February 13, 2025
X. A standby equity purchase agreement with Indaptus Therapeutics (INDP), publicly disclosed shortly after
These deals weren’t random. Yorkville’s dual investment gives it direct exposure to two sides of the same immunologic loop:
• NWBO, which owns the immune delivery system (DCVax + Eden) • Indaptus, which supplies the microbial immune booster (DECOY10/20)
In May 2025, Indaptus further announced a new offering and public company presentation highlighting the mechanism of action for DECOY20 and its clinical trial pipeline, including NCT05651022. With Yorkville holding a significant equity position and purchasing rights through the new financing structure, it effectively owns a pathway into the “danger signal” component of NWBO’s therapeutic matrix.
The strategic implication is clear: Yorkville is not a passive financier. It now holds a personal financial stake in one of NWBO’s most viable immune boosting agents. From a capital structure perspective, this alignment supports:
• Internal licensing flexibility between companies • Optional coordinated deployment under Specials • Future positioning for regulatory linked immune signal expansion
🧬 NWBO: Platform and Delivery
NWBO has developed DCVax, a dendritic cell based immunotherapy platform protected by exclusive patents and licenses covering the preparation, maturation, and administration of autologous dendritic cells. It trains the immune system using tumor lysate, most commonly autologous in DCVax-L. Exploratory trials using pooled or shared lysate libraries have been studied separately, but are not part of the DCVax-L platform. Its safety and systemic activation profile have been validated in Phase X glioblastoma trials led by Dr. Linda Liau.
In 2020, NWBO acquired Flaskworks and with it, the Eden platform, a closed system, sensor equipped cell therapy manufacturing unit designed for decentralized, GMP compliant, point of care deployment. Eden allows for:
• Automated, programmable DCVax production • Modular input selection (lysate, boosters, synthetic payloads) • Regulatory adaptability under frameworks like the UK’s SI XX
NWBO’s system is not a static treatment. It is a responsive, immune interface.
From the NYAS 2025 presentation, Dr. Marnix Bosch introduced a booster code matrix (e.g., IAV, IABD, IAVCBD) that maps to cytokine outputs, immune targets, and site specific strategies, designed to enable modular therapy based on tumor type, booster compatibility, and clinical setting.
This labeling logic, if paired with Eden, transforms DCVax from a fixed product into an adaptable immunologic framework. The Bosch presentation served as both a biological mechanism map and a regulatory calibration system. Because each code corresponds to a predefined immune profile, NWBO can now deliver different variants of DCVax under real world conditions without submitting an entirely new marketing authorization each time. The labeling becomes functional and adaptive. Calibration replaces rigidity. This essentially makes DCVax universal, applicable across tissue types, indications, and delivery sites.
💥 Indaptus: Danger Signal Amplification
Indaptus Therapeutics is developing DECOY10 and DECOY20, killed, nonreplicating strains of E. coli engineered to mimic microbial danger signals. Their mechanism of action includes TLR4 and NFκB activation, type I interferon production, and broad cytokine release across innate and adaptive compartments.
These constructs are designed not as therapies but as immune ignition payloads that provoke systemic immune activation. DECOY agents simulate infection through bacterial pattern recognition receptor pathways, triggering innate and adaptive responses. In studies presented at AACR 2024, SITC 2023, and published in Frontiers in Immunology (2024), DECOY10 consistently outperformed monospecific TLR agonists like Poly I C, R848, CpG, and LPS across key cytokines: GM CSF, IFNγ, IL 12p70, TNFα, and MIP1α.
This cytokine signature has now been clinically validated. In 2025, Indaptus completed the validation phase of its Phase 1b/2 trial (NCT05651022), evaluating DECOY20 in combination with BeiGene’s PD X inhibitor Tislelizumab. The trial confirmed immune activation and cytokine induction at therapeutically relevant levels with a clean safety profile. DECOY20 is now advancing to the next stage as a clinically qualified, system ready D class input.
This assignment directly maps to the Bosch booster matrix, introduced during NWBO’s NYAS 2025 presentation, which categorizes immune adjuvants as follows:
A class: Viral mimics (e.g. Poly I C, TLR3) B class: TLR4 ligands (e.g. G100, synthetic lipid A) C class: TLR7/8 agonists (e.g. R848) D class: Microbial danger signals (e.g. DECOY10/20) I class: Immune tone recalibrators (e.g. IFNγ) V class: Viral ignition (e.g. V937)
DECOY10’s cytokine output spans Bosch’s entire logic grid: GM CSF for dendritic cell maturation, IFNγ and IL 12p70 for T cell polarization and cytotoxic programming, TNFα for inflammation and tumor antigen release, and MIP1α for APC and lymphocyte recruitment. Functionally, DECOY10 extends well beyond the D class, overlapping A, B, C, and I booster functions.
This cross-functional profile is what makes DECOY uniquely suited for Eden, NWBO’s closed-system, programmable cell therapy manufacturing unit. Eden allows real time customization of DCVax formulations by site and patient using modular combinations like IAV, IABD, or IAVCBD. DECOY10 could simplify this structure by collapsing multiple inputs into a single cartridge, streamlining operations while preserving regulatory continuity under frameworks like SI XX.
Yorkville Advisors appears to have anticipated this. In July 2025, following Indaptus’ trial milestone, Yorkville provided an additional XXX million dollars via convertible note, with a X percent interest rate, XXX percent warrant coverage, and a XX month maturity. Full terms were disclosed here:
This was not bridge capital. It was strategic positioning. Yorkville had already financed NWBO in February 2025. Now, with equity exposure to both Indaptus and NWBO, Yorkville controls both ignition and delivery. According to market participants, Yorkville may convert early to secure equity ahead of broader institutional recognition. That urgency reflects conviction. DECOY20 is no longer a development-stage adjuvant. It is now an activated, clinically validated booster aligned with Eden’s real world deployment logic.
Yorkville’s NWBO note further supports this. It was structured not as secured debt, but as a convertible position, implying confidence in NWBO’s regulatory and commercial trajectory. The willingness to hold equity rather than debt, combined with the Indaptus structure, positions Yorkville across the entire immunotherapy system: signal (DECOY), engine (Eden), and output (DCVax).
With Eden’s programmable chassis, DECOY10 and DECOY20 can be deployed not only as D class inputs, but functionally across IAV, DIV, or IABD-coded formulations. In cold tumors, fibrotic lesions, or inoperable sites, DECOY’s multipathway output can replace multiagent regimens with a single, site-customized immune ignition.
This is not a single company story. It is a coordinated platform architecture.
DECOY delivers the signal. Eden executes the calibration. DCVax delivers the outcome. Yorkville is positioned across all three.
🧪 Merck’s V937: Viral Signal and Oncolytic Synergy
Merck’s V937 (Coxsackievirus A21) is a live, replication competent oncolytic virus designed to lyse tumor cells and stimulate local and systemic immune activation. It was acquired by Merck through its $XXX million purchase of Viralytics.
V937’s mechanism of action includes:
• Direct tumor cell lysis • Release of tumor associated antigens • Activation of pathogen recognition receptors including RIG I, MDA5, and TLR3 • Induction of type I interferon signaling • Promotion of immunogenic cell death (ICD) through release of DAMPs such as dsRNA, HMGB1, ATP, and calreticulin
V937 has been tested in multiple combination trials with pembrolizumab (Keytruda) in solid tumors (e.g. NCT02565992, NCT03323492), where it has shown the ability to transform cold tumors into immunologically active environments.
Importantly, NWBO’s XX K disclosed a Material Transfer Agreement in place since mid 2024 for a booster agent developed by a large pharmaceutical company, currently undergoing internal evaluation. The timing, classification, and delivery logic make V937 a strong candidate for this role. V937 remains under Merck’s control, and any use by NWBO would likely follow a licensing or limited scope agreement under Specials or future combination trials.
As a V class booster in NWBO’s framework, V937 could serve to precondition tumors prior to DCVax Direct injection, amplifying antigen spread, recruiting antigen presenting cells, and enhancing dendritic cell priming in vivo.
🧩 Functional Roles in NWBO’s Booster Matrix
From NWBO’s NYAS 2025 presentation, Slide X outlines a modular classification of dendritic cell booster types that can be combined in programmable sequences:
• A, B, C: TLR ligands • I: Immune modulators (e.g., IFNγ, checkpoint pathway agents) • D: Danger signals (e.g., DECOY10/20) • V: Viral pattern mimics (e.g., Merck’s V937)
These categories are deployed singly or in rational combinations (e.g., IAV, IABD, IAVCBD) to enhance specific immune functions. Each code combination corresponds to a unique immunological strategy: increasing TNFα and IL-12p70, shifting T cell polarization, promoting chemotaxis, or overriding tumor-induced suppression.
🎯 A B C Now Explicitly Mapped (Easy as X X 3)
The A, B, and C classes are now identifiable and anchored in both immunology and industry lineage:
• A = Poly I:C (TLR3):
A synthetic double-stranded RNA analog that mimics viral infection and drives type I interferon signaling through TLR3. Its most clinically relevant form is Poly ICLC, stabilized with carboxymethylcellulose and poly-L-lysine. This formulation is known commercially as Hiltonol, developed and manufactured by Oncovir, Inc.
Hiltonol has been used in numerous dendritic cell vaccine trials, including the Phase X glioblastoma combination trial with DCVax-L, whose results were published in Nature Communications in March 2024. That study showed a greater than XX% survival benefit for newly diagnosed glioblastoma patients treated with DCVax-L plus Hiltonol compared to historical controls. It validated the systemic priming power of Poly ICLC and reinforced the cytokine logic behind Bosch’s IAV/IABD-type booster profiles.
• B = G100 (TLR4):
A synthetic lipid A analog that activates TLR4 with significantly reduced toxicity compared to LPS. G100 was developed by Immune Design and acquired by Merck & Co. It has been used in multiple early-phase cancer trials, particularly intratumorally in lymphoma, and is known for driving local cytokine production and dendritic cell activation in vivo. Its inclusion in NWBO’s Eden-compatible matrix suggests Merck-derived TLR4 logic is interoperable with DCVax-Direct formulations, particularly in D/V/A-coded combinations.
• C = R848 (Resiquimod, TLR7/8):
R848 is a potent imidazoquinoline compound that activates TLR7 and TLR8, primarily in monocytes, dendritic cells, and macrophages. It was developed by MedImmune and is now owned by AstraZeneca. R848 has been used as a topical immune stimulant and as an injectable adjuvant in preclinical and early-phase cancer immunotherapy. It induces IFNα, IL-12, and TNFα and plays a complementary role to Poly I:C by targeting different subsets of innate cells. Its Th1-skewing and dendritic cell-activating effects make it a natural fit for Eden’s programmable IAV/IAC-type profiles, especially in combination with danger signals (D) or checkpoint modulators (I).
This classification is not arbitrary. These agents are not just immune potentiators, they are patented strategic assets. Their integration into NWBO’s Eden-compatible system implies molecular and operational interoperability with technologies controlled by Merck, AstraZeneca, Amgen, and Oncovir.
If true, that represents a quiet but profound structural alignment: one that bridges next-generation immune activation with legacy pharma control points, all layered into a modular, programmable system that NWBO now appears to own.
🧪 Plug and Play Boosters: Merck (B class), AstraZeneca (C class), Amgen (I class)
Merck: G100 (TLR4, B class)
G100 is a synthetic lipid A analog that activates TLR4 with reduced toxicity. Originally developed by Immune Design and now owned by Merck, it has been used intratumorally in early phase oncology trials. In the Eden matrix, G100 serves as a B class booster and pairs well with microbial or immune modulatory co-signals.
AstraZeneca: R848 (TLR7/8, C class)
R848 (Resiquimod), acquired through AstraZeneca’s 2007 acquisition of MedImmune, is a potent TLR7/8 agonist capable of triggering IFN alpha, IL-12, and TNF alpha. It activates plasmacytoid dendritic cells and supports Th1 polarized immunity. As a C class input, R848 complements Poly I C by expanding Eden’s reach across innate sensing pathways.
Amgen: Actimmune (IFNγ, I class)
Actimmune is the GMP formulation of interferon gamma (IFNγ), originally developed by Horizon Therapeutics and now owned by Amgen. Approved for chronic granulomatous disease and osteopetrosis, it has been incorporated into immunotherapy combinations as a classical biological response modifier. In Bosch’s NYAS 2025 presentation, IFNγ was explicitly referenced in the “Rescue of Low Producers” slide, where it converted suboptimal dendritic cell responses into high output cytokine profiles. It defines the I class: immune tone modulation and dendritic cell reactivation.
Each plug-and-play agent maps directly to Bosch’s A–I classification system, Merck’s G100 as B, AstraZeneca’s R848 as C, and Amgen’s IFNγ as I, demonstrating functional interoperability across the Eden framework.
🧬 Modular Architecture and Regulatory Significance
Each class plays a role in immune engagement:
• A B C: Synthetic innate immune activators, precisely titratable and GMP compatible • D: Microbial danger signals like DECOY10/20, broad spectrum activators of NFκB and type I IFN • V: Viral oncolytics like V937, releasing tumor antigens and danger signals in situ • I: Immune modulators that shape cytokine tone and checkpoint responsiveness (e.g., IFNγ, see Amgen’s Actimmune)
What Bosch presented was more than a biology slide. It was a modular labeling system. The code matrix is designed to function within the UK’s SI XX framework, allowing real time formulation switching (per patient, site, or protocol) without resetting the regulatory baseline.
In essence, DCVax has become relabelable by immune logic.
This flexibility, paired with Eden’s ability to execute customized formulations at the point of care, turns NWBO’s therapy from a static product into a universal programmable immunologic framework.
This modular framework depends on execution systems like Eden to ensure real-time precision and regulatory alignment, especially as global standards evolve.
⚙️ Flaskworks and Eden: The Integration Layer
Flaskworks’ Eden system is not just a device. It is the execution layer of NWBO’s entire immunologic logic. While the booster matrix (A to I) defines the immune inputs, Eden delivers the output: patient-specific, GMP-grade DCVax formulations calibrated to site, tumor, and signal.
It breaks the manufacturing bottleneck that has historically prevented cell therapy from scaling. More than that, it redefines the regulatory perimeter. Eden enables NWBO to swap in new boosters, adjust formulations, and apply localized immune strategy, all without reauthorizing the core DCVax platform.
This is made possible by Eden’s unique features:
• Per patient, per site, per booster configurability • Full traceability and immune signature documentation • Programmable logic built to match regulatory frameworks like SI XX • Rapid adaptation to local tumor immune environments (e.g., IAV at one site, IABD at another)
Plugged into Eden, NWBO can now integrate:
• DECOY20 as a D class microbial adjuvant (Indaptus) • V937 as a V class viral igniter (Merck) • G100 as B class (Merck) • R848 as C class (AstraZeneca) • Actimmune as I class (Amgen)
Each of these once stood alone as an investigational adjuvant. Now, through Eden, they can become programmable subroutines in a living immunologic engine. That shift, from product to platform, is what makes Eden central not just to NWBO, but to the future of decentralized immunotherapy.
💼 Strategic Implications and Yorkville’s Position
Yorkville is not just financing both companies. It has become one of the most prominent institutional holders of Indaptus and a direct capital partner to NWBO. With follow-on purchasing rights and exposure to the DECOY platform through Indaptus’ equity agreement, Yorkville has secured a cross-platform foothold:
• It can influence or broker booster integration between DECOY and DCVax • It has visibility into both manufacturing logic and immune signal design • It has potential positioning leverage as Specials transition into trial-based programs
This alignment gives Yorkville more than just capital exposure. It gives it strategic optionality across one of the most modular and programmable cell therapy architectures in oncology.
What makes DECOY20 unique, compared to synthetic agonists or legacy immunomodulators, is its ability to deliver a complete microbial danger signal in a single payload. It combines TLR4 engagement, NFκB activation, and type I interferon release into one broad-spectrum immune ignition event. Where synthetic inputs like G100 or R848 act on specific receptors, DECOY20 triggers a cascade, mimicking natural pathogen cues and potentially awakening immune responses in tumors that resist conventional priming.
This is likely why Yorkville chose to anchor itself here. DECOY20 is not an accessory; it’s a keystone signal. One that becomes exponentially more powerful when paired with a delivery engine like DCVax and a modular chassis like Eden.
Would it make sense for Yorkville to become a large investor in Indaptus if DECOY20 had no downstream partner or clinical use case? Probably not. Its standalone activity is promising, but its systemic value emerges through integration. As Eden evolves to accommodate real-world input variation, DECOY20 becomes a dynamic programmable booster that plugs directly into a modular therapeutic engine.
The value is not abstract. It is functional. And Yorkville is embedded on both ends of that function.
And as for the old toxic loan trope, Yorkville’s behavior makes the opposite case. Its continued investment, the increasingly favorable terms of its most recent offering, and its cross-platform exposure suggest a partner that is betting on this ecosystem to scale, succeed, and lead.
As Eden brings real-world adaptability to DCVax, DECOY20 becomes a live signal in a modular therapeutic engine. The value is not theoretical. It is functional. And Yorkville is positioned across both ends of that system.
🔚 Final Thought
If DECOY20 is D, V937 is V, Eden is programmable, and the TLR class is now explicitly populated with Poly I:C (TLR3), G100 (TLR4), and R848 (TLR7/8), then NWBO is no longer competing over whose agent is superior. It is architecting the immune stack that lets them all connect.
This is not a therapy built around a molecule. It is a programmable interface for immune logic, one that incorporates the best available danger signals, viral ignition tools, innate calibrators, and checkpoint enablers into patient-specific formulations.
Each module engages the immune system from a distinct axis: • D class: microbial danger • V class: viral ignition • A B C class: synthetic innate agonists • I class: immune recalibration
And all of it is controlled through Flaskworks’ Eden: a GMP-grade, traceable, and relabelable manufacturing platform designed not to produce one treatment, but to adapt many. It enables calibration by site, by signal, or by strategy, without resetting the regulatory baseline.
The Bosch booster matrix is not just a scientific diagram. It is a control system: a regulatory schema that formalizes immune orchestration under frameworks like SI XX. In that context, NWBO isn’t competing with pharma. It is programming it.
This is not a trial. It is a system. And Yorkville may already see it.
Bosch’s presentation is the gift that keeps on giving.💎
#Flaskworks #EdenSystem #DCVax #DECOY20 #V937 #Immunotherapy #CellTherapy #BoschMatrix #ModularMedicine #PrecisionMedicine #RealWorldEvidence #CDMO #CheckpointTherapy #TME #PointOfCare $MRK $AMGN $AZN $ABBV $LLY $VRTX $AMZN

XXXXX engagements
Related Topics signals $indp $nwbo convergence