[GUEST ACCESS MODE: Data is scrambled or limited to provide examples. Make requests using your API key to unlock full data. Check https://lunarcrush.ai/auth for authentication information.]  Ozmosi [@OzmosiHealth](/creator/twitter/OzmosiHealth) on x 2613 followers Created: 2025-07-18 16:04:40 UTC $SWTX European Commission conditionally approves EZMEKLY® (mirdametinib) for treating NF1-PN in adult and pediatric patients. More Info: $XBI $IBB $XPH $PPH 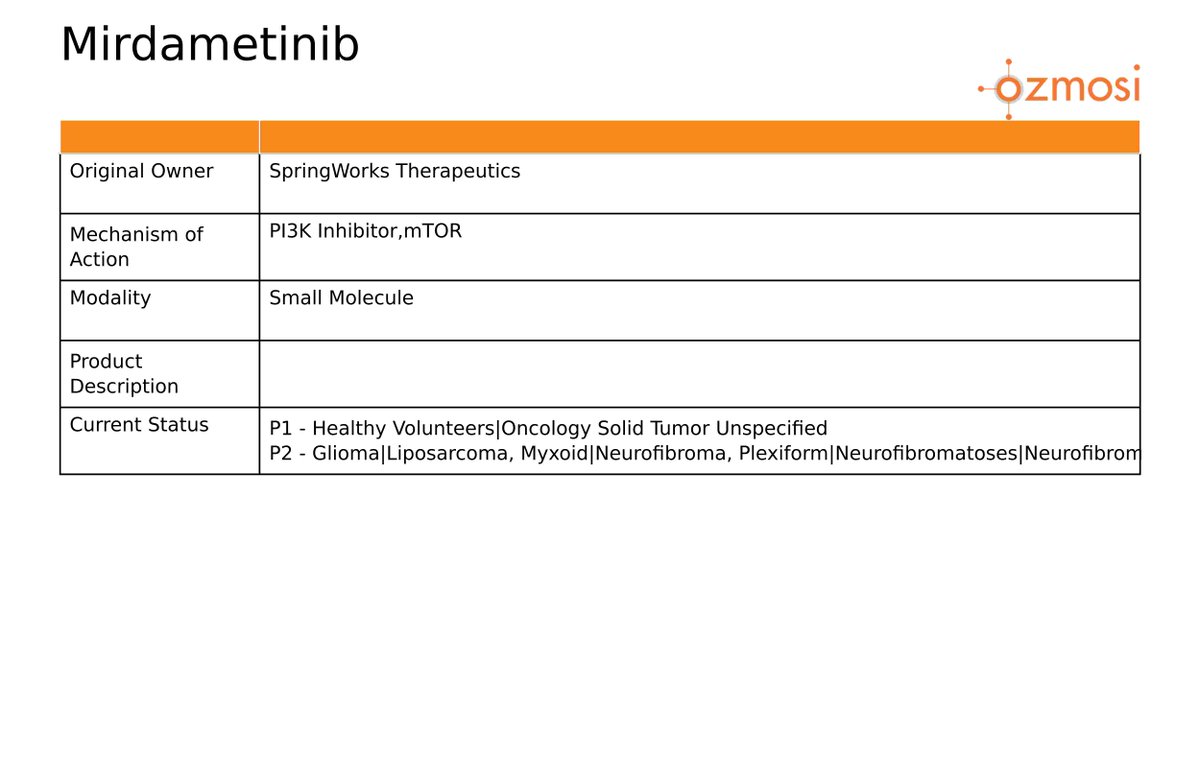 XXXXX engagements  **Related Topics** [$pph](/topic/$pph) [$xph](/topic/$xph) [$ibb](/topic/$ibb) [$xbi](/topic/$xbi) [more info](/topic/more-info) [$swtx](/topic/$swtx) [Post Link](https://x.com/OzmosiHealth/status/1946239709015085116)
[GUEST ACCESS MODE: Data is scrambled or limited to provide examples. Make requests using your API key to unlock full data. Check https://lunarcrush.ai/auth for authentication information.]
 Ozmosi @OzmosiHealth on x 2613 followers
Created: 2025-07-18 16:04:40 UTC
Ozmosi @OzmosiHealth on x 2613 followers
Created: 2025-07-18 16:04:40 UTC
$SWTX European Commission conditionally approves EZMEKLY® (mirdametinib) for treating NF1-PN in adult and pediatric patients.
More Info: $XBI $IBB $XPH $PPH

XXXXX engagements