[GUEST ACCESS MODE: Data is scrambled or limited to provide examples. Make requests using your API key to unlock full data. Check https://lunarcrush.ai/auth for authentication information.]  Ozmosi [@OzmosiHealth](/creator/twitter/OzmosiHealth) on x 2642 followers Created: 2025-07-15 20:32:29 UTC $KALV KalVista Pharmaceuticals has received UK MHRA approval for EKTERLY® (sebetralstat), the first and only oral on-demand treatment for Hereditary Angioedema. More Info: $XBI $IBB $XPH $PPH 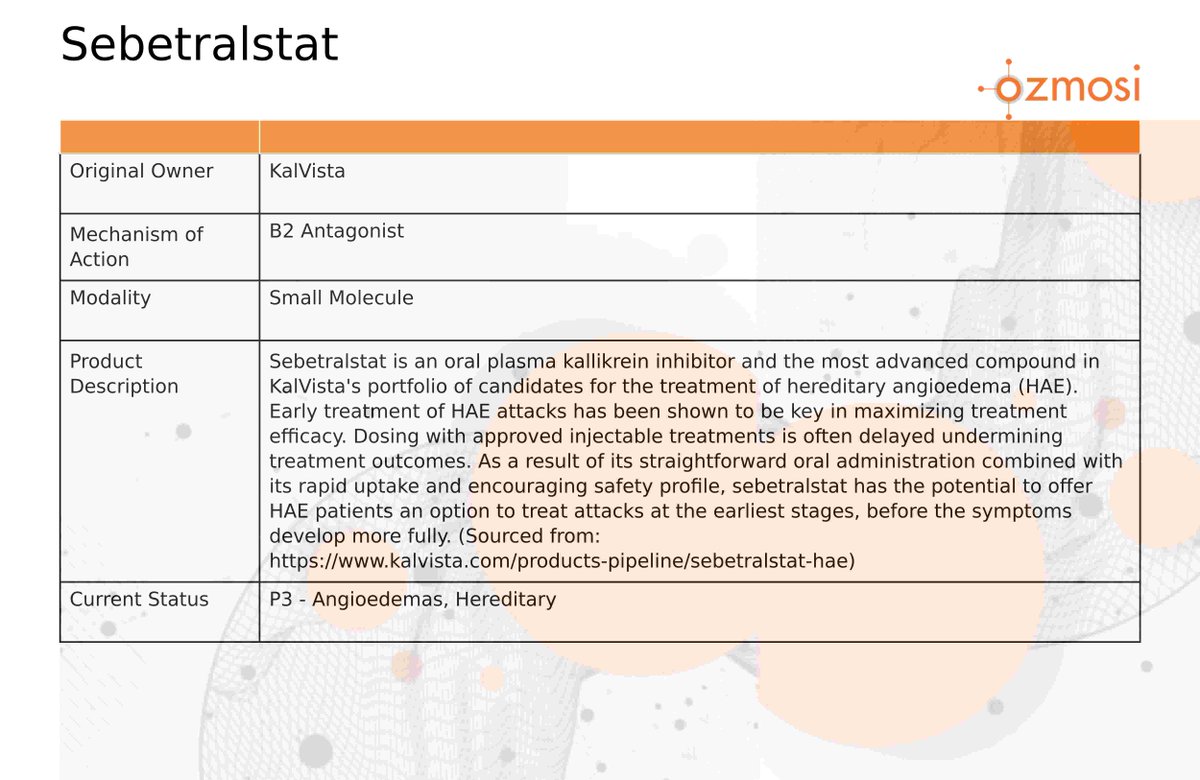 XXX engagements  **Related Topics** [$pph](/topic/$pph) [$xph](/topic/$xph) [$ibb](/topic/$ibb) [$xbi](/topic/$xbi) [more info](/topic/more-info) [$kalv](/topic/$kalv) [Post Link](https://x.com/OzmosiHealth/status/1945219941680210053)
[GUEST ACCESS MODE: Data is scrambled or limited to provide examples. Make requests using your API key to unlock full data. Check https://lunarcrush.ai/auth for authentication information.]
 Ozmosi @OzmosiHealth on x 2642 followers
Created: 2025-07-15 20:32:29 UTC
Ozmosi @OzmosiHealth on x 2642 followers
Created: 2025-07-15 20:32:29 UTC
$KALV KalVista Pharmaceuticals has received UK MHRA approval for EKTERLY® (sebetralstat), the first and only oral on-demand treatment for Hereditary Angioedema.
More Info: $XBI $IBB $XPH $PPH

XXX engagements