[GUEST ACCESS MODE: Data is scrambled or limited to provide examples. Make requests using your API key to unlock full data. Check https://lunarcrush.ai/auth for authentication information.]  Gene Investing w/Anthony 🧬 [@GeneInvesting](/creator/twitter/GeneInvesting) on x 13.6K followers Created: 2025-06-16 14:57:26 UTC Common questions by @PersimmonTI My reply: 1️⃣ Hemophilia A ($BMRN's Roctavian) •Initial FVIII levels look good, but rapid decline over time is well documented. •Pivotal trial showed median FVIII expression dropped from XX IU/dL at year X to ~24 IU/dL by year 2, and the trend continues downward. •Many patients require re-initiation of prophylaxis after a few years. •Durability concerns are a major reason for slow uptake post-approval. 2️⃣ NTLA-2002 is gene knockout via CRISPR-Cas9, not viral vector-based gene addition. That means no episomal DNA, no reliance on hepatocyte turnover, and theoretically, permanent suppression of kallikrein. One-time, durable, and clean mechanism of action. 3️⃣ No Immunosuppression Unlike AAV gene therapies for hemophilia that often require immune suppression (e.g., $QURE, $SGMO), Intellia’s platform has shown no need for steroids or prophylactic immunosuppression — a huge win in terms of tolerability and scalability. 4️⃣ Unmet Need Remains in HAE, Even Post-KALV Yes, $KALV and $PHVS are strong competitors, but they’re chronic and likely expensive. A one-time curative therapy that safely drops attack rates to zero or near-zero is highly differentiated and what patients truly desire. 5️⃣ Simplified Path to Adoption vs. Hemophilia Hemophilia gene therapy uptake has been sluggish due to safety baggage/screening process, complex factor replacement history, and entrenched physician/patient habits. HAE patients are typically younger, diagnosed early, and highly motivated for a functional cure. 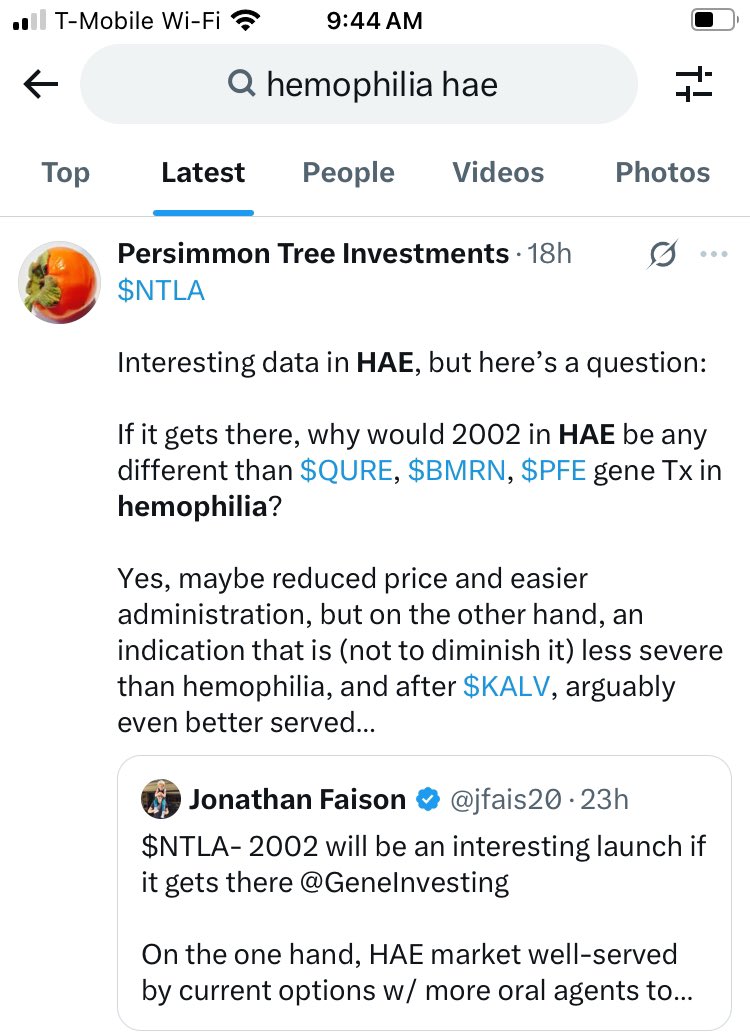 XXXXX engagements  **Related Topics** [$bmrns](/topic/$bmrns) [$reymi](/topic/$reymi) [investment](/topic/investment) [Post Link](https://x.com/GeneInvesting/status/1934626375131967717)
[GUEST ACCESS MODE: Data is scrambled or limited to provide examples. Make requests using your API key to unlock full data. Check https://lunarcrush.ai/auth for authentication information.]
 Gene Investing w/Anthony 🧬 @GeneInvesting on x 13.6K followers
Created: 2025-06-16 14:57:26 UTC
Gene Investing w/Anthony 🧬 @GeneInvesting on x 13.6K followers
Created: 2025-06-16 14:57:26 UTC
Common questions by @PersimmonTI
My reply:
1️⃣ Hemophilia A ($BMRN's Roctavian)
•Initial FVIII levels look good, but rapid decline over time is well documented. •Pivotal trial showed median FVIII expression dropped from XX IU/dL at year X to ~24 IU/dL by year 2, and the trend continues downward. •Many patients require re-initiation of prophylaxis after a few years. •Durability concerns are a major reason for slow uptake post-approval.
2️⃣ NTLA-2002 is gene knockout via CRISPR-Cas9, not viral vector-based gene addition.
That means no episomal DNA, no reliance on hepatocyte turnover, and theoretically, permanent suppression of kallikrein. One-time, durable, and clean mechanism of action.
3️⃣ No Immunosuppression
Unlike AAV gene therapies for hemophilia that often require immune suppression (e.g., $QURE, $SGMO), Intellia’s platform has shown no need for steroids or prophylactic immunosuppression — a huge win in terms of tolerability and scalability.
4️⃣ Unmet Need Remains in HAE, Even Post-KALV
Yes, $KALV and $PHVS are strong competitors, but they’re chronic and likely expensive. A one-time curative therapy that safely drops attack rates to zero or near-zero is highly differentiated and what patients truly desire.
5️⃣ Simplified Path to Adoption vs. Hemophilia
Hemophilia gene therapy uptake has been sluggish due to safety baggage/screening process, complex factor replacement history, and entrenched physician/patient habits. HAE patients are typically younger, diagnosed early, and highly motivated for a functional cure.

XXXXX engagements
Related Topics $bmrns $reymi investment