[GUEST ACCESS MODE: Data is scrambled or limited to provide examples. Make requests using your API key to unlock full data. Check https://lunarcrush.ai/auth for authentication information.]
 Andrew Caravello, DO @andrewcaravello on x 1095 followers
Created: 2025-07-23 10:52:37 UTC
Andrew Caravello, DO @andrewcaravello on x 1095 followers
Created: 2025-07-23 10:52:37 UTC
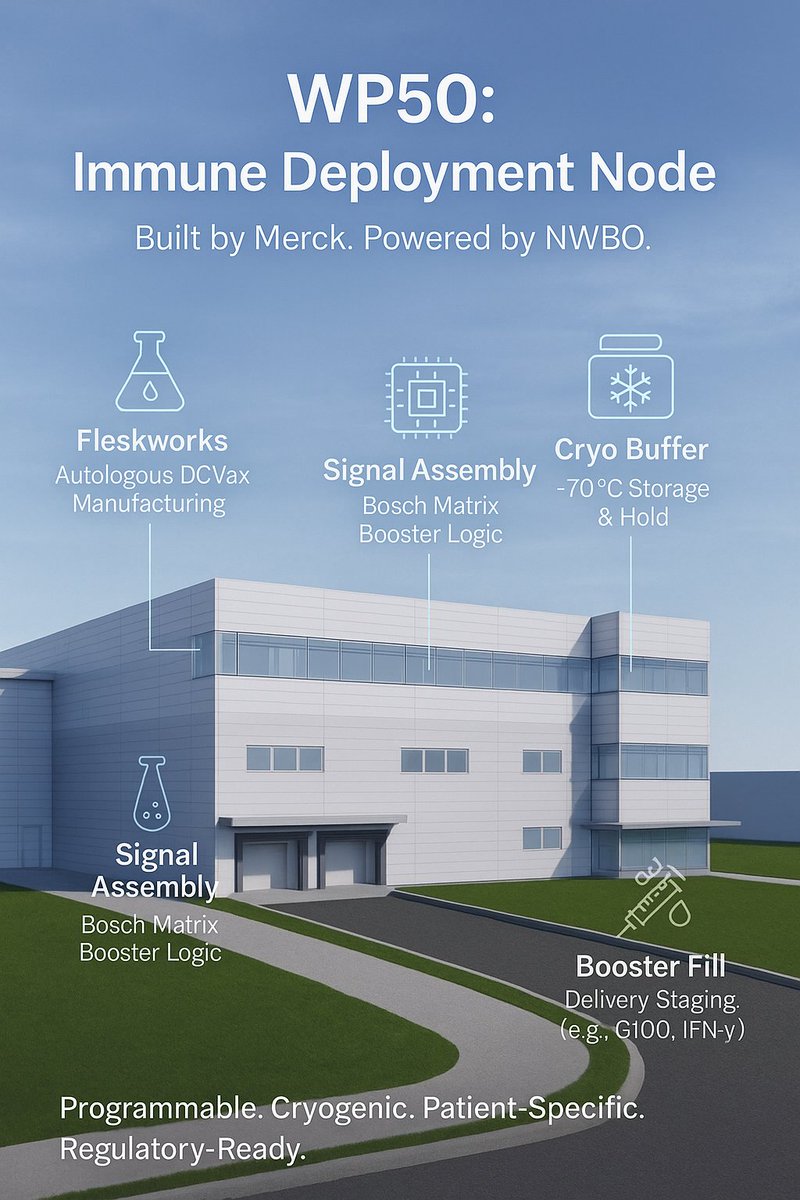
🧬 How $MRK WP50 Became the Hidden Deployment Hub for $NWBO Programmable Immune System
What began as a “vaccine plant” is now the staging ground for modular, patient-specific immunotherapy kits, built to deploy, not mass-produce.
⏱️ Estimated Read Time: 24-28 minutes
🏗️ The Facility That Quietly Changed Everything
In early 2021, as global headlines focused on mRNA, Merck quietly broke ground on a project that had nothing to do with COVID, flu, or Gardasil. The filing called it “Building 50” — WP50 in internal terms — and labeled it a “vaccine and office” expansion at the company’s massive West Point campus in Pennsylvania.
But behind that generic description was a $XXX million facility unlike anything else in Merck’s vaccine empire.
WP50 wasn’t designed for bulk manufacturing. It was built for precision staging — specifically, for modular immunotherapy kits. No batch fermenters. No large-volume bioreactors. And critically, no design elements to support high-throughput conjugate vaccine production, like what Merck uses for Prevnar or MMR.
Even its legal footprint gave it away. WP50 received a stormwater discharge exemption — a regulatory carve-out typically reserved for low-effluent, cleanroom-intensive sites like biologic dispatch facilities. Its projected wastewater output was just XXXXX gallons a day. That’s less than a commercial laundromat.
Why would Merck invest that much into a facility that doesn’t produce at scale?
Because it wasn’t built to output product. It was built to input logic.
🔬 A Facility Built for a Therapeutic Reboot
The timing was no coincidence.
In 2021, three key developments redefined the landscape of personalized immunotherapy:
•DCVax-L had just published its long-awaited glioblastoma survival results in JAMA Oncology
•DCVax-Direct completed Phase I, showing measurable tumor regression across multiple solid tumors with direct injection
•Mayo Clinic had begun releasing trial data showing that traditional conjugate vaccines like Prevnar and Pneumovax could enhance dendritic cell therapy when injected into tumors
Simultaneously, Northwest Biotherapeutics (NWBO) acquired Flaskworks — a Boston startup developing fully automated dendritic cell manufacturing hardware — and licensed IP from Roswell Park covering microbial adjuvants and DC maturation.
In short:
The architecture for a logic-coded immune platform had emerged. And WP50 looked built to support it.
Its layout — sterile fill rooms, cryogenic bays, and modular cleanroom segmentation — points to one thing: a real-time immunotherapy staging facility, not a traditional vaccine plant.
This is where the immune code gets deployed.
❄️ Cryo as a Design Principle — Not a Feature
Most vaccine plants operate with refrigeration between 2°C and 8°C. Even mRNA requires only −80°C at most. WP50 was designed for sub–150°C cryogenic staging — the range needed for autologous cell therapies like DCVax.
That kind of deep freeze isn’t a convenience. It’s a commitment.
It means the facility is architected to handle living cell products that must stay frozen from the moment of manufacture to the moment of injection — no room temperature hold, no thaw until delivery.
That matches:
•DCVax-L, cryopreserved and patient-specific
•Poly-ICLC, a lyophilized RNA mimic that benefits from cryogenic buffering pre-fill
•IFN-γ, DECOY20, and other Matrix-class boosters that require temperature integrity
This isn’t speculative. It’s architectural logic.
Merck didn’t just install cryo bays for flexibility. They embedded them as a core design element — because the kit they were building wasn’t generic.
It was meant to be customized. And cold. And ready to run.
🧬 Not a Fill Plant — A Signal Router
On its second floor, WP50 houses modular cleanrooms configured not by product line, but by signal class — the logic-coded categories that correspond to booster types in the Bosch Matrix.
Each suite can be isolated by function, allowing WP50 to:
•Fill lyophilized viral mimics like Poly-ICLC (Class A)
•Prepare sterile liquid emulsions like G100 (Class B)
•Load recombinant cytokines like IFN-γ or IFN-α2 (Class I)
•Co-package pre-filled syringes like Prevnar (Class V), filled nearby at Merck’s Building 63A
•Pair killed microbial agents like DECOY20 (Class D) or depot-formulated adjuvants like R848 (Class C)
This isn’t a production line. It’s a programmable stack execution node.
When Flaskworks manufactures a batch of DCVax for a patient, WP50 doesn’t just hold the vial. It fills or stages the corresponding immune boosters, labels the kit, and prepares it for personalized dispatch.
DCVax is the payload. WP50 is the switchboard.
🏛️ B32 — The Vault That Buys Time
Every high-speed node needs a buffer. That’s where Merck’s Building XX (B32) comes in — a dedicated −70°C storage facility integrated into the same West Point grid as WP50.
Its job isn’t to produce. Its job is to pause.
If Flaskworks finishes manufacturing but regulatory clearance isn’t ready, B32 holds the cryopreserved vaccine vials. If Eden scripts a booster stack that requires coordination, B32 holds the partially assembled kit until pairing is complete. If a trial site needs rolling inventory — B32 stages, tracks, and preserves.
The layout includes:
•Walk-in cryogenic vaults •Mobile ultra-cold units •GMP-grade chain-of-custody logs •Batch-linked barcode tracking
This isn’t warehouse storage. It’s deployment buffering for a living therapy that can’t afford to degrade.
Together, WP50 and B32 form a two-node immune engine:
•One that executes. •One that preserves.
🔁 SI XX and CNPV: The Legal Rails That Activate the Grid
Even the most sophisticated system is useless without regulatory alignment. WP50 doesn’t operate in isolation. It sits squarely between two real-world legal frameworks that allow its logic-coded kits to move.
🇬🇧 SI XX – UK Specials Law
•Allows named-patient use of unlicensed autologous cell therapies
•Supports pairing with GMP-grade boosters not yet centrally authorized
•Enables import/export of cryopreserved DCVax kits into the UK
•No marketing license required — just physician justification and GMP documentation
🇺🇸 CNPV – Commissioner’s National Priority Voucher (FDA)
•Enables 60–180 day review for therapies with completed Phase III trials
•Accepts patient-specific, real-world–ready formats
•Requires no new trial if safety/efficacy are proven — which they are for DCVax-L (via JAMA Oncology)
•Compatible with Flaskworks production and WP50 fill logic
This means Merck’s West Point stack is already regulatory-operable on both sides of the Atlantic.
In one mode, WP50 assembles and ships kits into the UK under SI XX. In another, it stages and dispatches them domestically under CNPV clearance.
In both, it bypasses traditional drug launch infrastructure — and moves the immune system at patient speed.
🧬 From Tissue to Therapy: A Personalized Immune Stack in Motion
This isn’t drug delivery. It’s logic delivery.
Every DCVax kit begins not in a factory, but with a patient’s own biology — a tumor sample and a monocyte harvest via leukapheresis.
There are two therapeutic pathways:
•DCVax-L, used post-resection for tumors like glioblastoma
•DCVax-Direct, used for inoperable tumors, with intratumoral injection paired with localized immune stimulants
In either case, the journey starts with living input. Not inventory. Not a generic dose.
And what comes next is automated.
⚙️ Flaskworks — The Engine That Manufactures Immune Code
Flaskworks isn’t a CMO or a contract lab. It’s a closed-system, GMP-enabled hardware platform — built to produce dendritic cell vaccines without human hands.
At its core is Eden, the automated device architecture described in U.S. Patent XXXXXXXXXX. Here’s what it does:
•Uses disposable cartridges to isolate, mature, and antigen-load monocytes
•Guides the cells through cytokine programming (e.g., GM-CSF, IL-4)
•Pulses them with tumor lysate — personalized per patient
•Cryopreserves the final DCVax product into single-use, barcoded vials
It tracks flow rate, pressure, pH, and maturation timing in real time — all logged and traceable.
Flaskworks isn’t batch manufacturing.
It’s immune programming — scaled, sealed, and per patient.
🧠 Eden — The Logic That Decides What Comes Next
Once Flaskworks produces the cell-based payload, Eden, NWBO’s internal orchestration system, assigns the booster logic using the Bosch Matrix — a functional immune codebook.
For each patient, Eden determines:
•Which signal classes are needed (A+B+I, or B+C+D, etc.)
•Which cytokines or immune tones are ideal (e.g., IL-12p70, IFN-γ)
•Whether the vaccine will be injected systemically (DCVax-L) or intratumorally (DCVax-Direct)
•What delivery formats are appropriate (syringe, patch, depot gel, etc.)
Eden doesn’t generalize. It scripts.
Then WP50 executes — physically pairing Flaskworks-made DCVax vials with logic-matched immune stimulants, all under regulatory guardrails.
No two kits are the same.
Each is a custom-coded immune program — generated from biology, compiled by Eden, and staged by Merck.
🧪 The Bosch Matrix: Functional Immune Classes That Plug In Like Code
In 2025, Dr. Marnix Bosch publicly outlined a new way to classify immune stimulants — not by molecule, but by signal function. This framework, now known as the Bosch Matrix, divides immune boosters into modular, combinable classes:
•Class A: Viral Mimics e.g., Poly-ICLC (Hiltonol) — lyophilized dsRNA that triggers TLR3, driving Type I interferons and IL-12p70 for systemic T-cell priming.
•Class B: Inflammatory Ignition (TLR4) e.g., G100 — a nanoemulsion used intratumorally to induce APC recruitment and localized inflammation.
•Class C: TLR7/8 Modulators e.g., R848, MEDI9197 — small molecules that fine-tune innate immune tone; may be formulated as depot injections or topical gels.
•Class D: Danger Signal Inducers e.g., DECOY20 — killed microbial ligands that activate PRRs broadly, often used for intratumoral shock priming.
•Class I: Cytokine Amplifiers e.g., IFN-γ or IFN-α2 — recombinant proteins used to restore signaling in low-responder or immunosuppressed patients.
•Class V: Recall Triggers e.g., Prevnar, Pneumovax — pre-filled conjugate vaccines originally used for infectious disease, now reimagined to awaken myeloid memory in cancer.
Each class isn’t a brand. It’s a function. Each agent isn’t just a drug. It’s a signal in a stack.
And WP50 was designed to physically execute every one.
🏭 WP50 Matches Format to Function
Each Matrix class has a specific physical format:
•Lyophilized (Class A, Class C) •Cold-chain liquid (Class B, Class I) •Sterile-filled microbial suspension (Class D) •Pre-loaded syringe (Class V)
WP50’s layout aligns precisely:
•Lyophilization lines to prep Poly-ICLC and TLR7/8 agents •Cold-fill bays for nanoemulsions and recombinant cytokines •Cryo staging rooms for DCVax vials and Poly-ICLC intermediates •Sterile prep suites for microbial adjuvants like DECOY20 •Labeling and co-packaging docks for Prevnar or Pneumovax syringes filled at B63/B63A
Each cleanroom suite can be dedicated to one signal class, ensuring sterility, format integrity, and per-class documentation.
That means WP50 can produce:
•A DCVax-L + Poly-ICLC + IFN-γ combo •A DCVax-Direct + G100 + DECOY20 pairing •Or a multi-stage DCVax-L regimen boosted over time with different Matrix agents
The building doesn’t run a pipeline.
It runs immune instructions, per patient.
💉 L vs. Direct: Route Matters — So Does Format
The booster logic isn’t just scientific. It’s spatial.
•DCVax-L is administered systemically, usually intradermally or subcutaneously, meaning boosters like IFN-γ or Poly-ICLC can be given separately, sometimes on offset schedules. Format flexibility is helpful but not always critical.
•DCVax-Direct, however, is injected into the tumor, often under image guidance. Boosters for Direct need to be:
•Pre-filled •Timed precisely •Co-localized with the injection
This makes sterile, syringe-ready boosters essential, especially for intratumoral agents like G100 or recall adjuvants like Prevnar.
That’s where Merck’s Building 63A completes the loop. Its XX million–dose annual capacity wasn’t built for DCVax itself. It was built to stage the booster fuel — for programmable, injectable therapy kits.
In short:
•DCVax is the driver. •The Bosch Matrix is the map. •WP50 and 63A are the vehicles. •And the destination is real-world immune control, on demand.
🌍 Kits Become Infrastructure: How the Immune OS Scales Without Rebuilding Pharma
In traditional biotech, scale means inventory — vials on shelves, pallets in warehouses, and global supply chains stretching across months.
But NWBO’s model scales differently.
Here, the therapy is logic-coded, and the supply chain is node-based:
•Flaskworks units produce immune payloads per patient •Eden scripts booster combinations using Matrix logic •WP50 stages and deploys kits per regulation •And B32 or equivalent cryo storage buffers demand
To replicate the system, you don’t need a new factory. You just need another node that speaks the same immune language.
🏗️ Flaskworks as the Hardware Platform, Eden as the Software Stack
Each Flaskworks unit is a sealed, GMP-certified immune factory:
•Small enough for hospital-based GMP suites •Cleanroom-ready for CMO or academic installs •Fully automated, running Eden’s logic scripts on disposable cartridges
And Eden doesn’t change across geographies. It speaks the same Bosch Matrix logic, Class A through V, no matter where it’s installed.
That means:
•A patient in London can receive DCVax-L + Poly-ICLC + IFN-γ from a Flaskworks unit at Advent
•A patient in Chicago can receive DCVax-Direct + G100 + DECOY20 from a node built at a local health system
The inputs vary.
The platform doesn’t.
It’s scalable, transferable, and globally regulatory-aligned.
✅ It’s a franchise model, for personalized immunity.
🛠️ Final Assembly: Why Merck’s Infrastructure Quietly Completes the Immune Stack
The real story of WP50 isn’t about vaccine volume. It’s about immune precision—and industrial enablement.
During COVID, Merck built WP50 and 63A to quietly house something the market hadn’t yet named: a programmable immunotherapy distribution system, routed by biology, not batches.
Today, it connects:
• Flaskworks: the engine that makes personalized DCVax from patient tissue
• Eden: the logic compiler that assigns Bosch Matrix–class boosters
• WP50: the facility that assembles, matches, and packages each stack
• B32: the vault that buys time, preserving each kit until dispatch
• 63A: the syringe engine that fills, stages, and scales real-world delivery boosters
• SI XX and UK Modular Manufacturing Law: the legal rails that now authorize decentralized, per-patient immunotherapy delivery
This is no longer theoretical.
As of July 23, 2025, the UK’s Human Medicines (Amendment) (Modular Manufacture and Point of Care) Regulations 2025 is now in force—a world-first legal framework enabling personalized cell and gene therapies to be manufactured, assembled, and administered near the patient.
The law supports:
• On-site and mobile production • Small-batch or single-patient dosing • Cryogenic handoff and direct dispatch • Regulatory control without re-approval
It is tailor-made for what WP50 already does.
And globally, regulators are catching up. The ICMRA’s international working group has now endorsed decentralized, point-of-care manufacturing as a viable and harmonizable pathway for advanced therapies, making WP50 not just a national asset, but a globally replicable immune infrastructure model.
“Highly personalised treatment—made for one person, in one place, at one time—becomes part of routine care,”
said MHRA Chief Executive Lawrence Tallon.
And this infrastructure isn’t operating in a vacuum.
While the U.S. has not yet enacted a formal decentralized framework, the CNPV (Commissioner’s National Priority Voucher) initiative shows signs of convergence, potentially offering expedited review for platforms like DCVax that already completed Phase III. If adopted, it would align the U.S. operationally with the UK’s legal breakthrough.
This system wasn’t built for Prevnar or Gardasil.
It was built for DCVax.
And it works with every Matrix-class booster, even those Merck doesn’t own, because the stack isn’t built around brands.
It’s built around immune function: signal mimics, TLR agonists, cytokine drivers, memory triggers.
What matters is not the molecule, but the message.
• Poly-ICLC? Check. • DECOY20? Check. • R848? G100? IFN-γ? Check, check, and check.
Merck owns the infrastructure. NWBO owns the code.
And together, they’ve quietly constructed something no one else has:
a plug-and-play immune execution engine, now legally operable in the UK, and structurally ready for the U.S. the moment policy catches up.
They didn’t just build a therapy. They built an operating system for live, logic-coded immunity.
It’s not theoretical. It’s running. And now, under law
It’s deployable. It’s scalable. It’s real. 🗽
$MRK $BMY $PFE $GILD $LLY $AZN $MRNA $VXRT $BGNE $INBX $MDCX $ONCS $MODV $VIR $REGN $SAGE $IOVA $TCRT $INDP $JNJ $NVS $SGEN $NVO #DCVax #Immunotherapy #CellTherapy #PersonalizedMedicine #CancerVaccine #DendriticCells #BoosterStack #Flaskworks #BoschMatrix #ProjectOrbis #ModularManufacturing #CryogenicStorage #SI87 #CNPV #Biotech #Oncology #ImmuneSystem
XXXXX engagements
Related Topics $nwbo hub $mrk stocks healthcare